ocrevus start up form
According to immunization guidelines live or live-attenuated vaccines should be administered at least 4 weeks prior to initiation of. A representative from OCREVUS Access Solutions or your.
Frontiers Multiple Sclerosis And Cancer The Ying Yang Effect Of Disease Modifying Therapies
These infusion reactions can happen for up to 24 hours after your infusion.
. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. These infusion reactions can happen for up to 24 hours after your infusion.
Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV. Genentech can start helping you when page 4 of this form is submitted by you or your doctors office in one of the following ways. Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS CONNECTS and begin receiving the services it.
There is a pregnancy exposure registry that monitors pregnancy and fetalneonatalinfant outcomes in women exposed to OCREVUS during pregnancy. Is this a new start or continuation of therapy. OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my access.
Once youve prescribed OCREVUS enroll your patients in OCREVUS Access Solutions. Send it via fax. Every 6 months infuse 600mg in 500mL of 09 NS.
If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy. RMS and PPMS and their open-label extensions up to. Prior Authorization Form for.
And sign and date the form or it could delay our ability to help you. Sample infusion referral form Please confirm compliance. Sign a printed form and fax or mail it to us or give it to your doctors office to do so Your doctor also has to fill out a form called the OCREVUS Start Form.
Prescription Enrollment Form. Instructions for Patients Please write legibly and complete all required fields on the OCREVUS Start Form to prevent delays. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with.
Once we have both. OCREVUS is a prescription medicine used to treat. To a final concentration of 12mgmL.
OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my access. Use this form to learn about your health insurance coverage and financial assistance options and enroll in optional services from Genentech Access Solutions. Swelling of the throat.
It is important that. The documents accompanying this transmission may contain confidential health information. Physicians are encouraged to.
Ocrevus ocrelizumab Fax completed form to 8086506487. Access Solutions is committed to helping your patients access the Genentech medicines they need providing assistance to your patients after OCREVUS is prescribed.

Multiple Sclerosis Symptoms And Causes Mayo Clinic

Experience With The Covid 19 Astrazeneca Vaccination In People With Multiple Sclerosis Multiple Sclerosis And Related Disorders
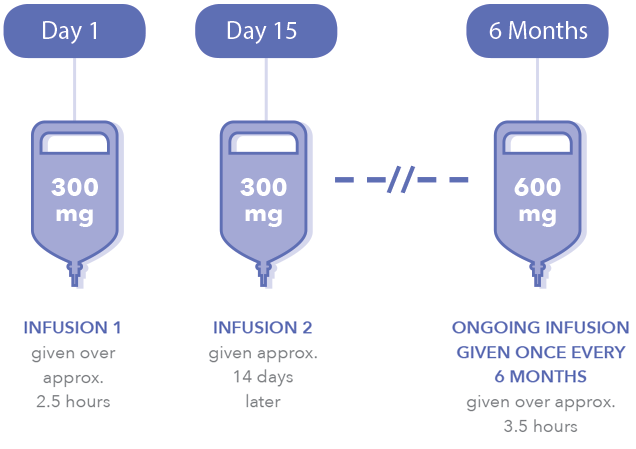
How Is Ocrevus Given Get On With Life
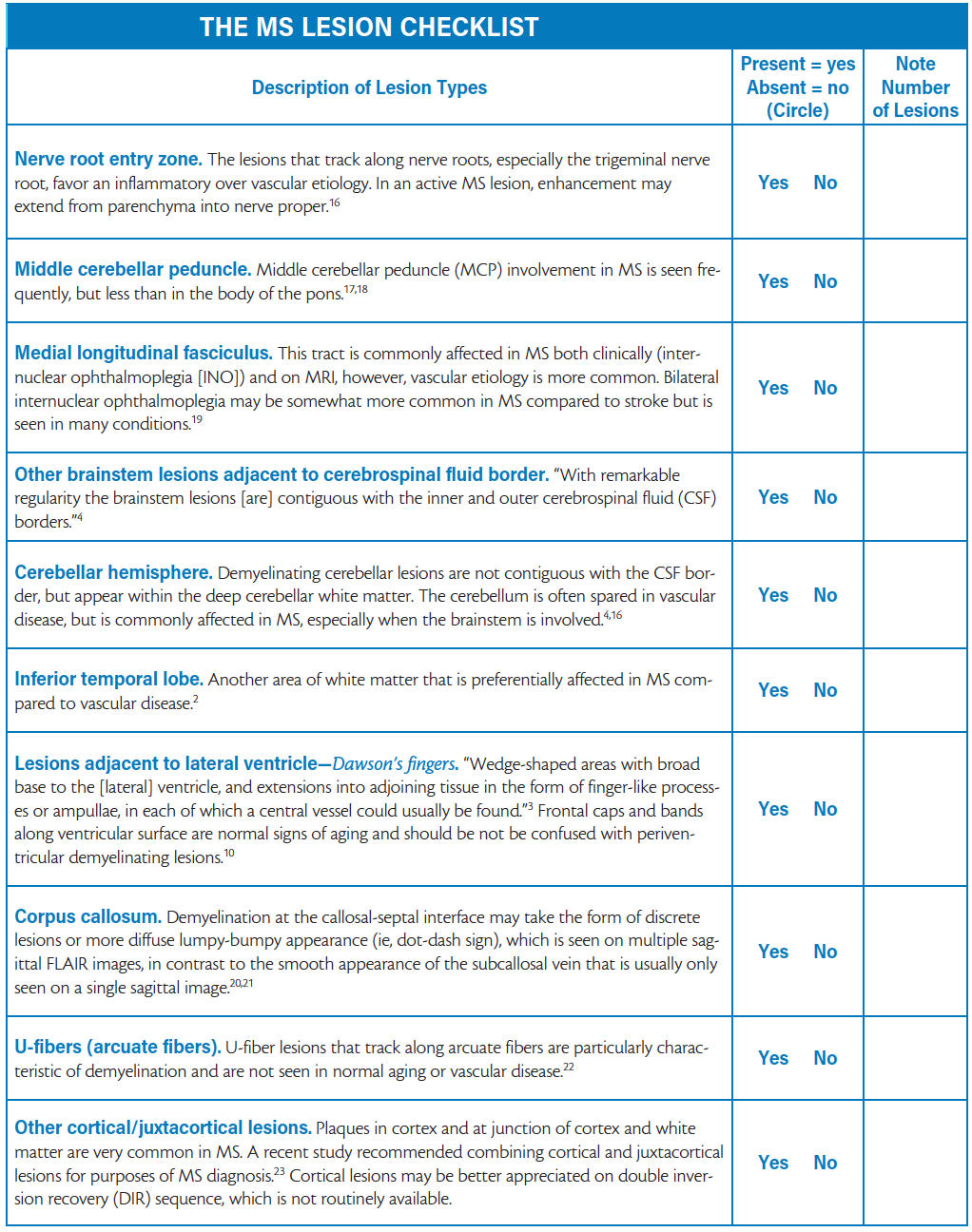
The Multiple Sclerosis Lesion Checklist Practical Neurology

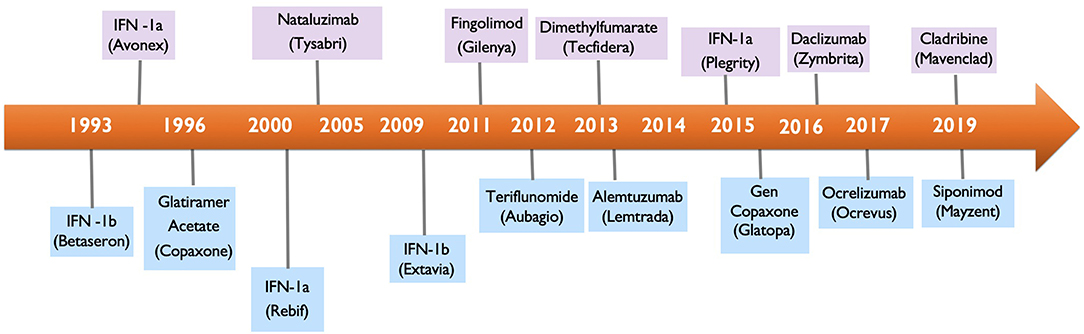
Multiple Sclerosis Two Decades Of Progress The Lancet Neurology

Ocrevus Ocrelizumab Multiple Sclerosis Ms Treatment
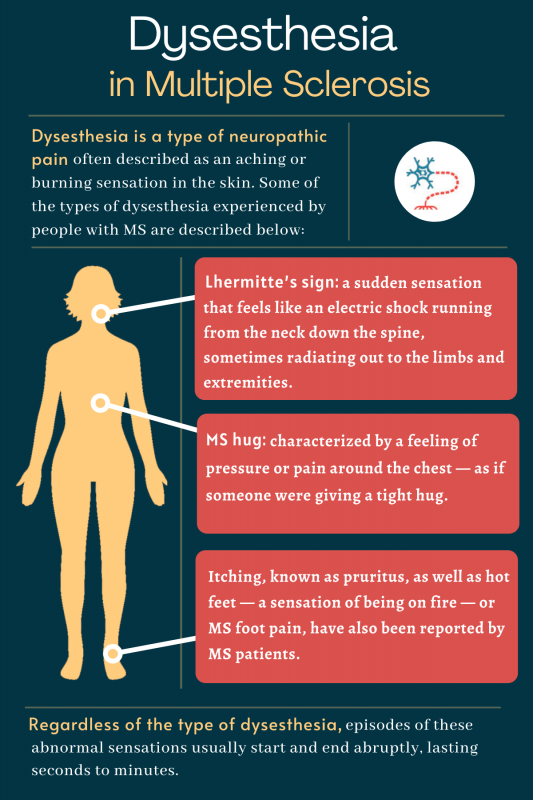
Dysesthesia In Ms Feeling Causes And Treatment Multiple Sclerosis News Today

Ocrevus Start Form Pdf Fill Online Printable Fillable Blank Pdffiller

The Future Of Multiple Sclerosis

Stluciefire On Twitter 5k Run Walk To Raise Funds And Awareness For Multiple Sclerosis March 21 In Tradition Https T Co Ikhfp3eedf Twitter

Multiple Sclerosis Fact Sheets Yale Medicine
![]()
Efficacy Of Avonex Interferon Beta 1a
Multiple Sclerosis Has A Common Viral Culprit Sparking New Approaches Science News

Most Impressive Drug Launch Roche S Ocrevus Biopharma Dive
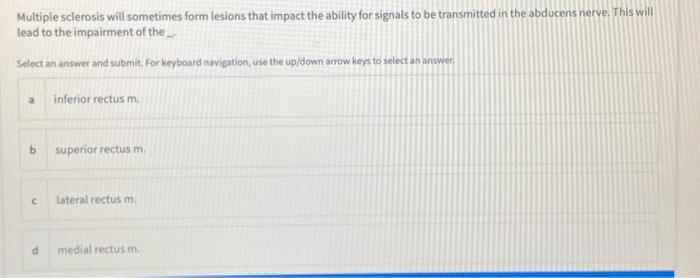
Solved Multiple Sclerosis Will Sometimes Form Lesions That Chegg Com

Roche S Ocrevus 2 Hour Infusion Time Gets Eu Approval For Ms Treatment S P Global Market Intelligence

Multiple Sclerosis The Plaque And Its Pathogenesis Nejm
:max_bytes(150000):strip_icc()/types-of-multiple-sclerosis-5200710_final-80ec48e459554962a559890ad290a980.jpg)
Types Of Multiple Sclerosis Ms Progression Outlook
What S The Difference Between A Brand Name Drug And A Generic Name Drug Goodrx